Abstract
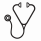
Introduction: Chronic kidney disease (CKD) is common in patients with sickle cell disease (SCD) and is associated with increased mortality. Current practice guidelines recommend treatment of albuminuria with angiotensin converting enzyme (ACE) inhibitors in hopes of slowing the decline in kidney function. Although short-term studies of ACE inhibitors show significant reductions in albuminuria, there are no published long-term studies of the consequences of treatment with these agents in patients with SCD. The purpose of this study was to evaluate the effect of ACE inhibitors/angiotensin receptor blockers (ARBs) on proteinuria and estimated glomerular filtration rates (eGFR) over time in adult patients with SCD. We also evaluated the effect of treatment with these renin-angiotensin-aldosterone system (RAAS) blocking agents on mortality.
Methods: We conducted a retrospective study of patients seen between January, 2004 and December, 2013 at an adult Sickle Cell Clinic. Clinical and laboratory variables were obtained from medical records. Proteinuria (at least 1+) was ascertained by dipstick urinalysis. Glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation. Patients had confirmed diagnoses of SCD, were at least 18 years old, and were evaluated during routine clinic visits. Mixed effects models were fit to ascertain the effect of RAAS blocking agents on proteinuria and eGFR over time among individuals who had a history of proteinuria. We included interaction terms between laboratory variables, time and treatment to evaluate the effect of these variables on the response to treatment over time, controlling for age and sex. Cox regression model was used for mortality analysis.
Results: Four hundred and forty seven patients with SCD (SS = 280, SC = 102, Sb0 = 22, Sb+ = 33, SE = 2, SLepore = 2, SD = 2, SHPFH = 4), median age of 28.5 years (IQR: 20 - 40 years) and 238 females (53%) were evaluated. The median duration of follow-up was 2.8 years (IQR: 0.99 - 6.3 years). Patients on RAAS blocking agents for proteinuria (N = 65) had significantly greater odds of proteinuria over time vs. patients with proteinuria but not receiving RAAS blocking agents (N = 112) (estimate: 0.87 [95% CI : (0.78, 0.96) 0.053], p = 0.008). No significant difference was seen in the decline of eGFR in patients on RAAS blocking agents vs. patients with proteinuria not receiving such treatment (estimate: 0.43 [SE: 0.31], p = 0.17). In age- and sex-adjusted univariate analysis for laboratory variables, higher baseline hemoglobin, hemoglobin F, WBC count, and lower baseline LDH were significantly associated with lower treatment effect on the rate of change in log-odds of proteinuria over time (Table 1). In the multivariable analysis, hemoglobin F was associated with lower treatment effect on the rate of change in log-odds of proteinuria over time (estimate: -0.077 [SE: 0.03], p = 0.012).
Presence of proteinuria at baseline was associated with an increased risk of death (HR: 2.74 [95% CI: 1.51, 5.0], p = 0.001). In addition, higher eGFR was associated with a lower risk of death (HR: 0.98 [95% CI: 0.97, 0.98], p < 0.0001). Treatment with RAAS blocking agents was not associated with a decreased risk of death (HR: 1.48 [95% CI: 0.38, 1.53], p = 0.33).
Conclusion: Treatment with RAAS blocking agents was not associated with a decline in eGFR, but was surprisingly associated with greater odds of proteinuria. Furthermore, although both proteinuria and decrease in eGFR were associated with an increased risk of death, treatment with RAAS blocking agents had no significant effect on the risk of death. Prospective studies are needed to evaluate the effect of RAAS blocking agents on progressive kidney disease in patients with SCD.
Ataga: Global Blood Therapeutics: Research Funding; Global Blood Therapeutics: Honoraria; Novartis: Membership on an entity's Board of Directors or advisory committees; Reprixys Pharmaceuticals Corporation (formerly known as Selexys Pharmaceuticals Corporation, which is not affiliated with Selexis S.A.): Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract